Ask an Expert
Back to Categories
What negative controls should be used for flow cytometry? Is there a preference for isotypic controls versus unstained cells?
Isotypic controls have been used in clinical labs since the advent of clinical flow cytometry and many labs are still using them daily. However, with the current, almost exclusive, use of directly conjugated monoclonal antibodies (Mab) in clinical flow labs, the use of isotypic controls are not recommended by the FDA in most situations.
Negative controls used for flow cytometry should ideally be cells of the same cell type, processed in the same manner and stained with the same antibodies minus the specific antibody-antigen component that needs the control such as FMO controls (Fluorescence Minus One). In the FMO method, cells are processed and stained in the same manner with all antibodies present in the tube except the antibody in question. The routine use of the FMO method for each antibody in a multicolor/multitube panel would be cost prohibitive. Isotypic controls are manufactured to match the immunoglobulin class, fluorochrome and mAb concentration of the specific antibody, but there has been much debate in the flow cytometry community over the last 2 decades about how accurately these controls show levels of non-specific binding within a test experiment.
Using populations internal in the experiment (tube) as a negative control has been found by a consensus of cytometrists, to be the most appropriate method to determine non-specific binding by the specific antibody. These cells are part of the experiment from the beginning and are exposed to all steps of processing and staining. For example, when staining for CD20 and CD3 in a lymphocyte population, there are cells which are negative for each antibody. The cells negative for CD20 are an appropriate negative control for the CD20 positive cells in the experiment. Within the same tube, the CD3 negative cells serve as an appropriate negative control for the CD3 positive cells. It can also be appropriate to use negative cells in another tube as long as tube is part of the same experiment and stained with the same fluorochrome and isotype.
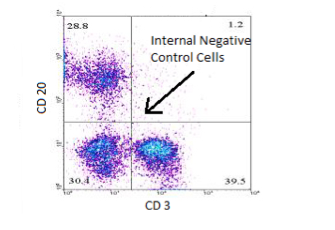
Running a tube of unstained cells can also be a helpful tool in evaluating any auto-fluorescence that may be on the cells. There are some situations in the clinical flow lab where isotypic/isoclonic controls may continue to be appropriate such as: intracellular staining, quantitative applications, rare event analysis, etc. However, the same diligence should be exercised in matching concentration, isotype, and fluorochrome in these controls as well.
Further Reading:
1. Hulspas R, O’Gorman MRG, Wood BL, Gratama JW, Sutherland DR (2009) Considerations for the control of background fluorescence in clinical flow cytometry. Cytometry B Clin Cytom. 76:355-364.
2. Keeney M, Gratama JW, Chin-Yee IH, Sutherland DR (1998) Isotype controls in the analysis of lymphocytes and CD34+ stem and progenitor cells by flow cytometry - time to let go! Cytometry. 34:280-283.
3. Roederer M (2002) Compensation in flow cytometry. Curr Protoc Cytom. 22:1.14.1-1.14.20.
Author: Julia Bass
